Revolutionizing the Imaging AI Marketplace: How AICentral.org updates help healthcare professionals


Christoph Wald, MD, PhD, MBA, FACR
Chair, ACR Commission on Informatics
Chair, Radiology, Lahey Hospital & Medical Center
Burlington, MA
In recent years, we have seen a rapid increase in the number of FDA-cleared imaging AI products available to augment radiology practice. Manufacturers have developed solutions across many organ systems, modalities and use cases, but it can be challenging to find the right product for a specific healthcare site and patient population.
Keeping up with this rapidly evolving marketplace and what it has to offer to imaging practices is a daunting task, especially if AI product selection relies on researching individual company’s websites and other available scattered online resources. For this reason, the ACR created AICentral.org. It is regularly maintained and updated as new products are approved. This online searchable directory continuously aggregates all commercially FDA-cleared products available to U.S. imaging practices. The newest AICentral.org updates make it easier than ever to navigate and find suitable imaging AI products. Practice leaders can review available products for a given use case they are interested in, or they can simply browse available solutions by modality, body part, type of disease/condition, FDA clearance category and so on.
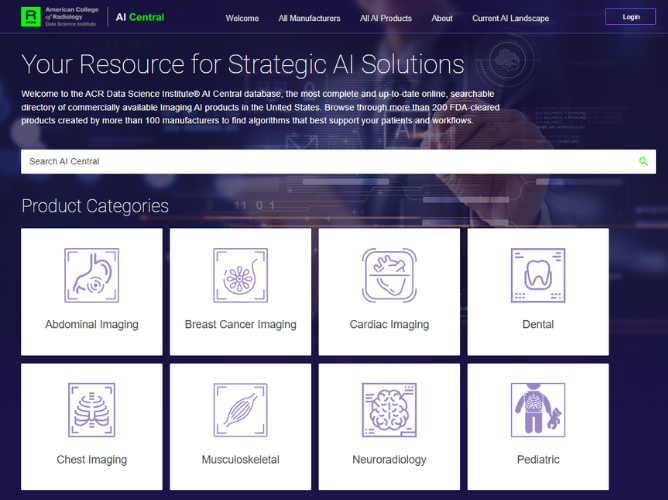
AICentral.org is also soliciting an unprecedented amount of information about algorithm development including scanner type, protocols and other technical parameters that influenced it, as part of the Transparent-AI program. Transparent-AI is a program that consists of a set of data elements voluntarily provided by imaging AI manufacturers who currently have an FDA-cleared product in the U.S. market. The data collected falls within general categories such as Model Identification, Characteristics, Indications for Use, Performance, Training Details and Limitations. Examples of data elements include intended user, age range, scanner manufacturer and scanner models used in stand-alone performance. Together with the so-called “instructions for use” (IFU) - an FDA-required manual each AI company must compile and share with customers - this provides a much-enhanced foundation for data-driven decision making about algorithm choice and licensing.
In our experience, clinical end users are not always aware that these IFU documents exist and may not have had a chance to review them either before purchasing and/or using a software. We strongly recommend reviewing the IFU for use of AI products in practice, and optimally before licensing a particular solution, because IFUs contain important information about standalone testing and performance of the AI, among other things. Reviewing this information can provide clues whether the AI is likely to perform well in the customer’s practice. ACR is soliciting the IFUs from manufacturers who are being asked to share these important documents for posting and easy access on AICentral.org.
 Manufacturers who participate in this program will receive the Transparent-AI badge on their product listing, allowing AICentral.org users to easily spot those who are participating in the program. Efforts to solicit all of this additional information are well under way and will continue throughout 2024 and beyond. Our goal is to optimally inform radiology practices so they can make the best decisions on suitable AI for their practices to enhance care for patients.
Manufacturers who participate in this program will receive the Transparent-AI badge on their product listing, allowing AICentral.org users to easily spot those who are participating in the program. Efforts to solicit all of this additional information are well under way and will continue throughout 2024 and beyond. Our goal is to optimally inform radiology practices so they can make the best decisions on suitable AI for their practices to enhance care for patients.
Christoph Wald, MD, PhD, MBA, FACR | Chair, ACR Commission on Informatics | Chair, Radiology, Lahey Hospital & Medical Center | Burlington, MA
Revolutionizing the Imaging AI Marketplace: How AICentral.org updates help healthcare professionals
-

You may also like
Unlocking AI Transparency in Radiology: A New Era with AICentral.orgFebruary 8, 2024 | Christoph Wald, MD, PhD, FACRAs radiologists, we strive to deliver high-quality images for interpretation while maintaining patient safety, and to deliver accurate, concise reports that will inform patient care. We have improved image quality with advances in technology and attention to optimizing protocols. We have made a stronger commitment to patient safety, comfort, and satisfaction with research, communication, and education about contrast and radiation issues. But when it comes to radiology reports, little has changed over the past century.