| 1 |
COVID-19 |
1.0 |
CT |
Help radiologists recognize the findings of COVID-19 infection as seen on chest CT exams and reduce variability in reporting these findings to referring providers. This is based on the four-category COVID-19 reporting system described by the joint RSNA, ACR, and STR consensus statement. |
Complete |
2020 Q2 |
COVID-19 |
| 2 |
LI-RADS |
v2018 |
CT/MRI |
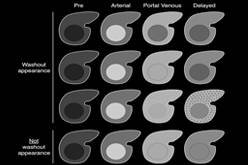
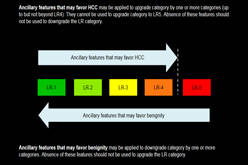
LI-RADS® was created to standardize the reporting and data collection of CT and MR imaging for hepatocellular carcinoma (HCC). |
Complete |
2020 Q3 |
LI-RADS |
| 3 |
TI-RADS |
v2017 |
US |
Thyroid nodules are exceedingly common, leading to costly interventions for many lesions that ultimately prove benign. |
Final Review |
2020 Q4 |
|
| 4 |
IF-PITUITARY LESION |
v2018 |
CT/MRI/FDG PET |
The ACR Incidental Findings Committee presents recommendations for managing pituitary findings that are incidentally detected on CT, MRI and 18F-fluorodeoxyglucose PET. |
Final Review |
2020 Q4 |
|
|
5
|
PI-RADS |
v2.1 |
MRI |
To improve early diagnosis and treatment of prostate cancer, ACR, AdMeTech Foundation and ESUR formed a joint effort to develop standards for the Prostate Imaging Reporting and Data System (PI-RADS®). |
Initial Review |
2021 Q1 |
|
| 6 |
O-RADS |
v2019
v2020
|
Ultrasound/MRI |
“O-RADS™” is an acronym for an Ovarian-Adnexal Imaging-Reporting-Data System which will function as a quality assurance tool for the standardized description of ovarian/adnexal pathology. |
Final Review |
2020 Q4 |
|
| 7 |
Lung-RADS |
v1.1 |
CT |
Lung-RADS® is a quality assurance tool designed to standardize lung cancer screening CT reporting and management recommendations, reduce confusion in lung cancer screening CT interpretations,and facilitate outcome monitoring. |
Final Review |
2020 Q4 |
|
| 8 |
NI-RADS |
v2019 |
PET/CT and MRI |
Neck masses, especially in the setting of previous treatment for head and neck cancer, can be very complex to interpret. |
Initial Review |
2021 Q1 |
|
| 9 |
IF-Adrenal |
v2017 |
CT |
The ACR Incidental Findings Committee presents recommendations for managing adrenal masses that are incidentally detected on CT or MRI. These recommendations represent an update to the adrenal component of the JACR 2010 white paper on managing incidental findings in the adrenal glands, kidneys, liver, and pancreas. |
Initial Review |
2020 Q4 |
|
| 10 |
BI-RADS |
5th edition |
Mammo, Breast US, Breast MRI |
BI-RADS reporting enables radiologists to communicate results to the referring physician clearly and consistently, with a final assessment and specific management recommendations. |
Under development |
2021 Q1 |
|
| 11 |
IF-Chest Mediastinal |
v2018 |
CT |
Recommendations for managing incidentally detected mediastinal and cardiovascular findings found on CT. |
Initial Review |
2020 Q4 |
|
| 12 |
IF-Liver |
v2017 |
CT |
These recommendations represent an update from the liver component of the ACR 2010 white paper on managing incidental findings in the pancreas, adrenal glands, kidneys, and liver. |
Initial Review |
2020 Q4 |
|
| 13 |
IF-Thyroid |
v2015 |
CTMRI/FDGPET |
The ACR formed the Incidental Thyroid Findings Committee to derive a practical approach to managing ITNs on CT, MRI, nuclear medicine, and ultrasound studies. |
Final Review |
2020 Q4 |
|
| 14 |
IF-Pancreas |
v2017 |
CT/MRI |
The ACR Incidental Findings Committee (IFC) presents recommendations for managing pancreatic cysts that are incidentally detected on CT or MRI. These recommendations represent an update from the pancreatic component of the JACR 2010 white paper on managing incidental findings in the adrenal glands, kidneys, liver, and pancreas. |
Initial Review |
2020 Q4 |
|
| 15 |
IF-Renal |
v2018 |
CT |
The ACR Incidental Findings Committee (IFC) presents recommendations for renal masses that are incidentally detected on CT. These recommendations represent an update from the renal component of the JACR 2010 white paper on managing incidental findings in the adrenal glands, kidneys, liver, and pancreas. |
Final Review |
2020 Q4 |
|
| 16 |
IF-Adnexal |
v2020 |
CT, MR |
The ACR Incidental Findings Committee (IFC) presents recommendations for managing adnexal masses incidentally detected on CT and MRI. These recommendations represent an update of those provided in previous JACR 2013 white paper. |
Under development |
2021 Q1 |
|
| 17 |
TBI-RADS |
N/A |
- |
- |
Planned |
2021 Q1 |
|
| 18 |
C-RADS |
Draft document in progress |
CT |
C-RADS establishes a standardized approach to the reporting of colorectal and extra-colonic finding in patients. |
Planned |
2021 Q1 |
|
| 19 |
BT-RADS |
New topic |
TBD |
- |
Planned |
2021 Q2 |
|
| 20 |
PE-RADS |
New topic |
CT |
- |
Planned |
2021 Q2 |
|
| 21 |
MSK-RADS |
New topic |
Bone (x-ray, CT, MR) |
- |
Planned |
2021 Q2 |
|
| 22 |
MSK-RADS |
New topic |
Soft Tissue Tumors (TBD) |
- |
Planned |
2021 Q2 |
|
| 23 |
IF-Pulmonary (Fleischner) |
v2018 |
CT |
Not an ACR product |
Planned |
2021 Q1 |
|
| 24 |
IF-Chest Nodules |
v2020 in progress |
CT |
Guidelines for the management of pulmonary nodules detected incidentally at CT examinations performed for purposes other than lung cancer screening. |
Planned |
2021 Q1 |
|
| 25 |
IF-Pineal |
New topic |
- |
- |
Planned |
2021 Q2 |
|